NCC-Bio and GR Boston N. Announce Breakthrough in Overcoming KRAS Therapy Resistance, Pancreatic Cancer Awareness Month
Breakthrough in Overcoming KRAS Therapy Resistance During Pancreatic Cancer Awareness Month, November 2025.
Together, we can make this the month the world learns how to turn resistance into response for pancreatic cancer.”
BOSTON, MA, UNITED STATES, November 1, 2025 /EINPresswire.com/ -- ________________________________________— Warren Park
NCC-Bio and Global Bio Match Service Announce Breakthrough in Overcoming KRAS Therapy Resistance During Pancreatic Cancer Awareness Month
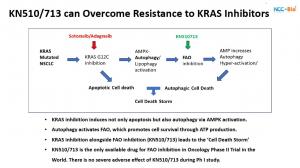
In recognition of Pancreatic Cancer Awareness Month, NCC-Bio (National Cancer Center Bio Co., Ltd.) and Global Bio Match Service (GBMS) announced new findings from their joint KN510713 program — the world’s first clinical-stage FAO inhibitor proven to prevent resistance to KRAS-targeted and cytotoxic therapies in solid tumors, including pancreatic cancer.
Pancreatic cancer remains one of the deadliest malignancies, with a five-year survival rate below 12%. Despite advances with KRAS inhibitors such as sotorasib and adagrasib, patients almost universally develop acquired drug resistance. KN510713 directly addresses this major unmet need.
“We discovered that all cancers — especially pancreatic — depend on fatty acid oxidation (FAO) to survive treatment,” said Dr. Soo-Youl Kim, CEO of NCC-Bio and Principal Scientist at the National Cancer Center, Korea. “By inhibiting FAO with KN510713, we can completely block the energy source that cancer cells use to escape KRAS inhibition, leading to what we call a ‘Cell Death Storm’.”
Presented at the 7th RAS-Targeted Drug Development Summit (Boston, Sept. 2025), Dr. Kim’s research demonstrated that combining KN510713 with KRAS inhibitors such as sotorasib or adagrasib significantly enhanced cancer cell death and prevented the recurrence of resistant clones in preclinical and xenograft models.
In a completed Phase I clinical study in advanced solid tumors, KN510713 achieved a 41.7% stable-disease rate with no dose-limiting toxicity, validating its safety and mechanism of action. The upcoming Phase IIa clinical trial in pancreatic ductal adenocarcinoma (PDAC) is expected to begin in November 2025.
“KN510713 represents a paradigm shift for the entire KRAS therapeutic landscape,” said Dr. Warren Park, CEO of Global Bio Match Service. “Our goal this month is to bring this science to the attention of potential partners, investors, and patient advocates who share the mission to finally stop pancreatic cancer resistance.”
NCC-Bio and GBMS are actively seeking strategic co-development or licensing partners among leading oncology pharmaceutical companies for global advancement of KN510713, as well as philanthropic and venture supporters committed to transforming pancreatic cancer outcomes.
Warren Park
GR Boston North
+1 978-269-4198
email us here
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.